Glass Transition Temperature (Tɡ): Definition, Significance and Factors Affecting
What is glass transition temperature?
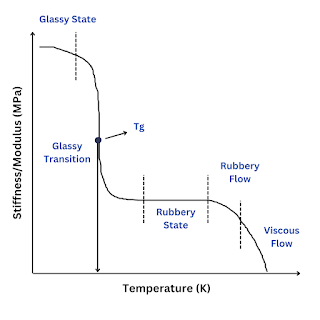
When plastic or rubber is cooled up to a certain temperature, it becomes so hard and brittle that it breaks into pieces on the application of stress. So, the temperature below which the polymer becomes hard, brittle, and glassy and above which it is softener and flexible is known as glass transition temperature (Tɡ).
The glass transition is a property of only the amorphous region of a semi-crystalline solid whereas the crystalline portion remains crystalline during the glass transition.
It is important to note that the transition does not occur suddenly at a unique temperature but rather over a range of temperatures. The temperature in the middle of the inclined region is taken as the Tɡ.
Significance of glass transition temperature (Tg)
- Glass transition temperature (Tɡ) is used as a measure for evaluating the flexibility of a polymer and the type of response the polymeric material would exhibit to mechanical stress.
- Glass transition temperature (Tɡ) tells us that, above it, a polymer will be soft and flexible and exhibit viscoelasticity (Delayed elastic response) and below it, a polymer will be hard and brittle and will possess dimension stability.
- The glass transition temperature (Tɡ) value along with the Tm value indicates the temperature region at which a polymeric material transforms from a rigid state to a soft viscous state. This helps in choosing the right processing temperature which is the temperature region in which the material can be converted into the finished products through different processing techniques such as molding calendaring and extrusion.
Factors affecting glass transition temperature (Tg)
1. Chain flexibility and rigidity
Stiffening groups in the polymer chain reduce the flexibility of the chain and increase the value of glass transition temperature (Tɡ). In other words, we can say that the greater the intrinsic chain flexibility, the smaller will be the value of glass transition temperature (Tɡ).
2. Intermolecular Forces
Stronger intermolecular forces due to dipole forces, H-bonding, etc decreases the mobility of the chain which increases the value of Tɡ. For example, Tɡ of PVC is greater than Tɡ of polypropylene because PVC has stronger intermolecular forces than polypropylene because of the dipole-dipole forces from the C-Cl bond.
3. Molecular weight
Glass transition temperature (Tɡ) is directly proportional to the molecular weight of the polymer that is when the molecular weight of the polymer increases, Tɡ also increases.
4. Pendant Groups
Pendant groups have a considerable amount of effect on chain mobility. Bulky pendant groups like benzene can catch on neighboring chains like a "fish hook" and restrict rotational freedom which increases the glass transition temperature (Tɡ).
For example, a Big group like the adamantyl group will not only act as a "fish hook" but also it will act as a "downright boat anchor" that will increase the Tɡ.
Tɡ of poly(ether ketone) with an adamantyl group is 225 ℃ which is greater than the Tɡ of poly(ether ketone) that does not have an adamantyl
group.
5. Cross-linking
The greater the degree of cross-linking, the higher will be the Tɡ because the presence of cross-links between chains restricts rotational motion.
6. Plasticizers
Plasticizers are low molecular weight compounds that are added to plastics to increase their flexibility and workability. This weakens the intermolecular forces between the polymer chains and increases the mobility thus decreasing the glass transition temperature (Tɡ).
7. Copolymerization
Random copolymers have lower Tɡ since it tends to promote the disorder, reduce molecular packing, and reduce the inter-chain forces of attraction.
Determination of Glass transition temperature (Tg)
Glass transition temperature (Tɡ) can be determined by various methods like thermal analysis, Dynamic mechanical behavior, Broad-line NMR, Dielectric loss method, and Differential scanning calorimetry (DSC).
The differential Scanning calorimetry (DSC) method is the most common method to calculate glass transition temperature (Tɡ).