Vulcanization of Rubber: Definition, Properties and Applications
What is Vulcanization of rubber ?
As we know that, natural rubber is soft and sticky and becomes even more so at high temperatures and brittle at low temperatures. Also, it is not resistant to the action of organic solvents and can be easily attacked by oxidizing agents.
So, to solve this problem, Charles Goodyear in 1839, found a process called Vulcanization, which can improve the physical and chemical properties of rubber.
Vulcanization is a process that consists of heating raw rubber with sulfur at 373-415 K temperature. Since this process is slow, therefore additives like zinc oxide are used to accelerate the rate of vulcanization.
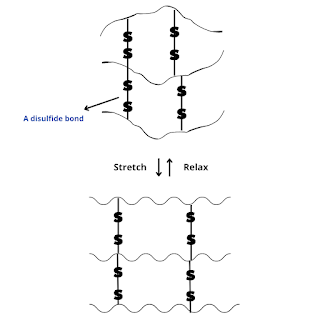
During vulcanization, sulfur bridges or cross-links between polymer chains are introduced either at their reactive allylic positions or at the sites of the double bonds.
These cross-links make rubber hard and stronger and remove the stickiness of natural rubber because the individual chains can no longer lip over but are instead locked together in a giant size molecule.
Properties of Vulcanized rubber
- Vulcanized rubber has excellent elasticity and a lower water absorption tendency.
- Vulcanized rubber is resistant to the action of organic solvents and oxidizing agents.
- Vulcanized rubber has a high degree of cross-linking, resulting in the high rigidity of the polymer.
- Vulcanized rubber has high tensile strength and high abrasion resistance.
Application of Vulcanized rubber
Applications of vulcanized rubber depend upon the amount of sulfur added.
- Rubber made with 1-3 % Sulphur is soft and stretchy and is used to make rubber bands.
- Rubber made with 3-10 % sulfur is more rigid and is used in the manufacture of tires.
- Rubber made with 20-25 % Sulphur is used for making ebonite.
- Rubber made with 30% sulfur is used for making battery case rubber.