Alizarin Dye: Definition, Synthesis, Properties and Application
What is Alizarin Dye?
Alizarin is a red dye having chemical formula C₁₄H₈O₄ which is generally used to dye cotton, wool, and silk.
Alizarin was originally obtained from the root of a common madder plant called Rubia tinctorum. It is also known as Turkey Red as it was best dyed in Turkey and in 1869, it becomes the first natural dye to be duplicated synthetically.
Synthesis of Alizarin
Following are some methods for the synthesis of alizarin:
1. From anthraquinone
The starting material for the synthesis of alizarin is an anthraquinone. It can be easily obtained by Friedel-crafts acylation of benzene with phthalic anhydride.
Anthraquinone is then sulfonated with concentrated sulphuric acid at a high temperature to give anthraquinone-b-sulphonic acid. Alizarin is obtained by fusion of anthraquinone-b-sulphonic acid with caustic soda.
2. By bromination of anthraquinone
Another synthesis is given by Graeve (in 1869). In this method, anthraquinone is brominated to yield dibromo anthraquinone. The obtained compound, when fused with caustic potash, gives alizarin.
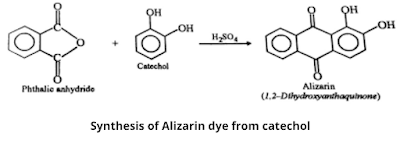
3. Synthesis from catechol
This is a new method for the synthesis of alizarin in which alizarin is obtained when catechol is condensed with the phthalic anhydride in the presence of anhydrous AlCl₃ or concentrated H₂SO₄ at 70 ℃ temperature.
Properties of Alizarin
1. Alizarin is a red crystalline solid having a melting point of 28.9-29.0 ℃.
2. It is insoluble in water and ethanol but soluble in hexane and chloroform.
3. It dissolves in alkalies to give a purple solution.
4. Alizarin is a pH indicator as it changes color depending on the pH of the solution.
Applications of Alizarin
1. It can be used as a mordant dye in which the resulting color varies with the nature of metal used for mordanting. Thus, we get a red color with aluminum, violet color with iron, and brown violet with chromium.
2. It is used as a purgative.
3. It is used for making printing inks.
4. It is used to dye cotton and wool.
5. In geology, Alizarin is used as a stain to differentiate the calcium carbonate minerals, especially calcite and aragonite.
Structure elucidation of Alizarin
1. Alizarin chemical formula is C₁₄H₈O₄.
2. On heating with Zn-dust, it gives anthracene.
From the above reactions of alizarin (conversion to anthracene and anthraquinone), it is clear that it is a derivative of anthraquinone.
3. The formation of disodium salt and diacetyl derivative suggests the presence of the 2-hydroxyl groups in alizarin.
4. On vigorous oxidation with strong oxidizing agents, it gives phthalic acid or its anhydride with no hydroxyl group. This shows that both the hydroxyl groups are present in the same ring.