Silicone Polymers: Definition, Preparation, Properties and Applications
What are Silicone Polymers?
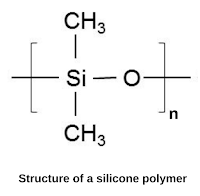
Silicones are polymeric organosilicon compounds containing (Si-O-Si) linkages and have the general formula (R₂SiO). They are typically colorless, oils, or rubber-like substances that may be linear, cyclic, or crosslinked.
Silicone polymers have very high thermal stability and are also called high-temperature polymers. They are widely used in sealants, adhesives, lubricants, medicine, and cooking utensils.
Preparation of Silicone Polymer
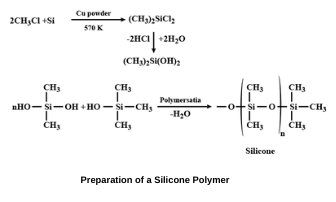
When methyl chloride reacts with silicon in the presence of copper as a catalyst at a temperature of 570 K, various types of methyl-substituted chlorosilanes are formed.
Hydrolysis of dimethyldichlorosilane that is (CH₃)₂SiCl₂ followed by condensation polymerization forms straight-chain polymers called silicone polymers.
Properties of Silicone polymer
1. It has low thermal conductivity, chemical reactivity, and toxicity.
2. They have low surface tensions and are capable of wetting most surfaces.
3. It does not support microbiological growth and can repel water.
4. It has high gas permeability and good electrical insulation properties.
Applications of Silicone Polymer
1. They are used in the manufacturing of sealants, surfactants, pressure-sensitive tapes, anti-foam agents, silicone rubber molds, etc.
2. They are used in medicinal and cosmetic implants due to low toxicity.
3. They are used as lubricants in both high and low temperatures.
4. They are used as grease, varnishes and these can be used even at low temperatures.