Poly (vinyl acetate): Structure, Preparation, Properties and Applications
What is Poly (vinyl acetate)?
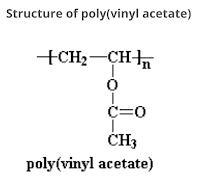
Poly (vinyl acetate) (PVA) was discovered in Germany in 1912 by Fritz Klatte. It is an aliphatic rubbery synthetic polymer with chemical formula (C₄H₆O₂)n which belongs to the polyvinyl ester family. It is also known as wood glue or Elmers's glue due to its adhesive properties for which it is used as adhesive for porous materials like wood, paper, and cloth.
Structure of Poly (vinyl acetate) (PVA)
Preparation of Poly (vinyl acetate) (PVA)
Poly (vinyl acetate) (PVA) is a vinyl polymer that is prepared by free radical vinyl polymerization of the monomer vinyl acetate.
Properties of Poly (vinyl acetate) (PVA)
1. PVA is an amorphous polymer having a glass transition temperature (T𝘨) between 30-45 ℃.
2. PVA offers good adhesion to most of the surfaces.
3. Degree of polymerization of PVA is between 100 to 5000 and it can be treated with alkali, which gradually results in polyvinyl alcohol and the alkali acetate.
4. PVA does not cross-link and it can be dissolved in many solvents other than water.
Application of Poly (vinyl acetate) (PVA)
1. Emulsified PVA is used in water-based adhesives like pastes and glues.
2. PVA can be used as a resinous component of latex paints, offering compatibility with a wide range of other paint chemicals.
3. PVA is used as a primer for drywall and other substrates.